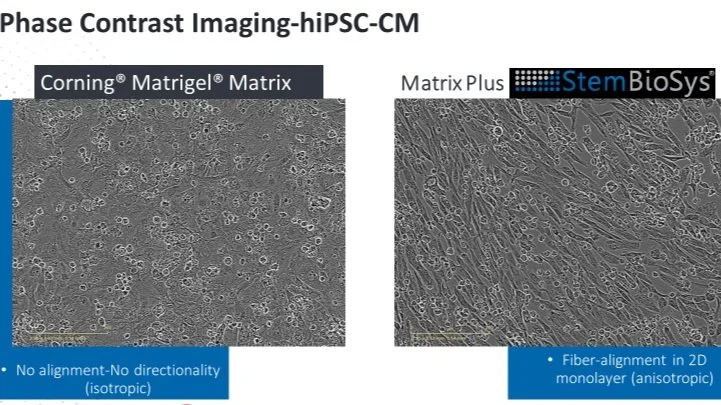
Mature Chamber Specific Human Cardiomyocytes for High Throughput Cardiotoxicity Testing
Human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs) can be generated in nearly unlimited quantities. hiPSC-CMs have been validated for use in drug discovery projects and are replacing the use of animals in many instances. However, limitations of this in vitro system have hindered widespread adoption of hiPSC-CMs for pre-clinical toxicity testing. One limitation is the immature phenotype of hiPSC-CMs. Here we present a human cell derived extracellular matrix (ECM) plating technology that solves this limitation by promoting the maturation of hiPSC-CMs in one week. This ECM is available commercially as CELLvo™ Matrix Plus from StemBioSys. Another limitation is the lack of chamber specificity of hiPSC-CMs. Here we present data showing the impact of using chamber specific hiPSC-CMs on the outcome of medication screening. We also introduce a new commercially available atrial specific hiPSC-CM product available from StemBioSys.
Thank you to our hosts at Reprocell.
View the recording now.