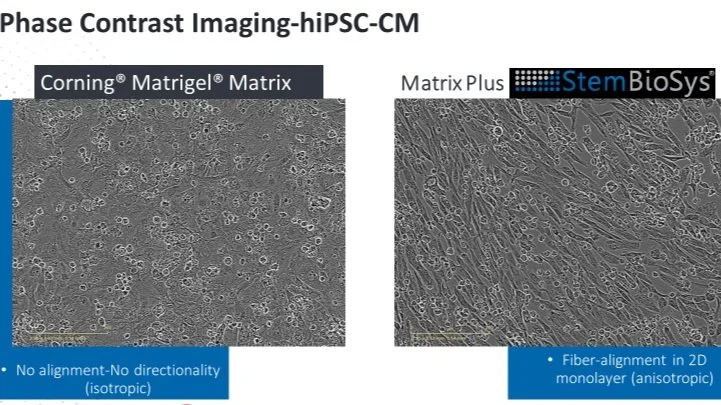
Topic: The FDA is now accepting In Vitro cardiotoxicity screening results from human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs) for investigational new drugs. Mature hiPSC-CMs are preferred in order to translate in vitro results to the adult human heart. Here we will review the use of StemBioSys’ human cell-derived extracellular matrix (ECM) CELLvo™ Matrix Plus for rapid and robust hiPSC-CM maturation using high throughput screening plates (96 well). Additionally, we will review the use of mature hiPSC-CMs for repeated measures of electrophysiological function via genetically encoded calcium indicator (GCaMP6). Repeated measures enable the ability to test the chronic effects of drugs on hiPSC-CM structure and function. Some medications can cause delayed onset toxicity that can only be measured in vitro using repeated measures. Data shows the utility of mature hiPSC-CM in vitro assays to determine toxicity occurring over the course of four days with measurements of intracellular calcium flux and monolayer confluence.
Presenters: Travis Block and Todd Herron.
Email us at info@stembiosys.com for more information.