For a PDF version of this technical bulletin, click here.
Introduction
Cartilage damage as a result of traumatic injury or long-term wear is the major cause of disability worldwide and a significant health priority for the rapidly aging global population. To better understand mechanisms of cartilage damage and repair, it is desirable that investigators be able to study chondrocytes in vitro.
While in vitro work with human cells is preferred to non-human cell lines, in order to improve relevance and translational potential of discoveries, major obstacles exist. Sources of human primary tissue for research are severely limited, and chondrocytes rapidly undergo dedifferentiation during in vitro expansion. In chondrocytes, dedifferentiation is marked by reduced expression of type II collagen (Col2) and an increase in expression of type I collagen (Col1). Col2 is the structural backbone of healthy cartilage and makes up more than 90% of collagen found in articular cartilage. In contrast, Col1 is present in very small amounts or is absent altogether but, is abundant in fibrocartilage. Together, these factors limit the availability of human chondrocytes possessing a biologically-relevant phenotype. Most commercially available chondrocytes have already been expanded and therefore have already sustained a significant change in their native phenotype. To catalyze progress in this space, StemBioSys® maintains a stock of commercially-available cryopreserved P0 chondrocytes. Furthermore, CELLvo™ Matrix has recently been shown to improve the isolation and expansion of human chondrocytes, while minimizing significant change in phenotype [1]. Here, we compare gene expression of CELLvo™ Human Articular Chondrocytes (HC-A) to other commercially available articular chondrocyte products. Additionally, we examine the effect of culture on substrate choice (CELLvo™ Chondrocyte-Derived (CD) Matrix (in development) vs. tissue culture plastic (TCP)) on chondrocyte gene expression. We show that CELLvo™ HC-A are the only chondrocytes with a native phenotype, and that CELLvo™ CD Matrix minimizes loss of phenotype during in vitro expansion.
These new tools have the potential to facilitate improved basic and translational research in this field.
Materials and Methods
Study Design
CELLvo™ HC-A were isolated from fresh articular cartilage and frozen as primary cells. For comparison, competitor cells were purchased from two large cell manufacturers at the lowest passage available (P1 and P2, respectively). Upon thawing, both CELLvo™ HC-A and competitor cells were prepared for gene expression analysis. Additionally, CELLvo™ HC-A and competitor cells were seeded on StemBioSys’ CELLvo™ CD Matrix and TCP for one passage. Proliferation, morphology and phenotype were compared.
Substrate and Media Preparation
CELLvo™ CD Matrix was prepared using protocols adapted from those previously described [2]. Briefly, HC-A were seeded onto fibronectin-coated plates. After being induced to secrete an extracellular matrix on the surface of the dish, the plates were decellularized using low concentration detergent. The remaining decellularized matrix was washed and then dried for future use.
Cell Culture
Cells were cultured in aMEM supplemented with 15% fetal bovine serum, 1% antibiotic/antimycotic, and 1% GlutaMax. Cells were seeded at 10,000 cells/cm2 and incubated at 37˚C with media changes every three days. When cells reached 90% confluence, they were trypsinized and counted using an automated cell counter.
Gene Expression
Gene expression was determined by reverse transcription polymerase chain reaction (RT-PCR). Immediately after thawing vials, approximately 5x105 cells per group were rinsed with cold (4°C) phosphate buffered saline and collected in cold (4°C) Trizol. RNA was isolated by phenol-chloroform extraction and 1 μg cDNA was synthesized from measured RNA. cDNA was diluted and mixed with TaqMan master mix and primers at a 2:3 ratio.
Results
CELLvo™ Primary HC-A exhibit a native phenotype compared to Competitor’s (passaged) HC-A
Native chondrocytes are identified based on their localization in cartilage and the expression of a high ratio of Col2 to Col1. The Col2/Col 1 ratio is often used as a surrogate for chondrogenic potential (ability to form cartilage matrix) and as the key marker of “healthy” chondrocytes [3]. To test the “quality” of commercially available chondrocyte products, the Col2/Col1 gene expression was measured immediately after thawing. CELLvo™ primary HC-A exhibited a Col2/Col1 ratio 37X higher than competitors (Figure 1). Most HC-A on the market today are sold after the first passage. However, after expansion most HC-A have already lost a significant portion of Col2 expression.
Figure 1. Gene expression measured by RT-PCR immediately after thawing. Gene expression was determined by the delta-delta Ct method. Expression of Col2 and Col1 was normalized to the housekeeping gene GAPDH.
CELLvo™ HC-A expanded on CELLvo™ Chondrocyte Matrix retained a better chondrocyte phenotype
To minimize costs and facilitate large studies, especially in vivo studies, it may be desirable to expand chondrocytes for one or more passages in order to achieve large cell numbers. Unfortunately, it is difficult to maintain cells that resemble native chondrocytes using traditional culture tools. Because extracellular matrices contain important cues for regulating cell behavior and phenotype [4], we expanded commercially available chondrocytes on CELLvo™ CD Matrix and TCP.
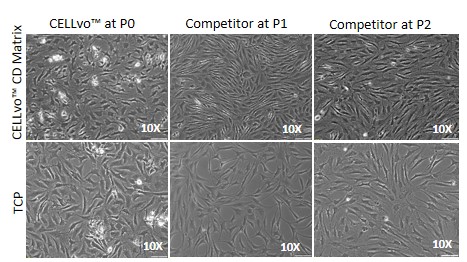
After thawing, only CELLvo™ HC-A had a high Col2/Col1 ratio (Fig 1) and only when cultured on CELLvo™ CD Matrix could those cells retain elevated Col2/Col1 after expansion. For all HC-A products, culture on CELLvo™ CD Matrix drastically increased proliferation rate (Table 1). However, only CELLvo™ HC-A showed stellate morphology (Fig 2) or decreased dedifferentiation (Fig 3) when cultured on CELLvo™ CD Matrix. The inability to detect phenotype retention in other cell products may be because the initial phenotype already had little resemblance to native chondrocytes.
Table 1. The fold of expansion (E1) was calculated as the average cell number at first passage divided by the average cell number seeded. The fold of expansion (E2) was calculated as the average cell number at second passage divided by the average cell number seeded. The total folds of expansion from start to finish= E1 x E2. The Population Doubling Level (PDL) was calculated using the formula: PDL=Log10(N/N0)x3.32 in which N=number of cells at the time of harvesting and N0=number of cells at the seeding time. PDL on each substrate was calculated using the average number of cells at first passage to the last passage.
Figure 2. Brightfield images of chondrocytes in culture on CELLvo™CD Matrix vs. TCP. CELLvo™ HC-A on CELLvo™ CD Matrix (top left), exhibit a stellate morphology typical of chondrocytes. All other groups exhibit a more spindle-like, fibroblast morphology.
Figure 3. Relative gene expression of cells after culture on CELLvo™ CD Matrix or TCP. Gene expression was determined by the delta-delta Ct method. Expression of Col2 and Col1 was normalized to the housekeeping gene GAPDH. Preservation of chondrocyte phenotype is often expressed as a ratio of Col2/Col1.
Discussion
In the present study, CELLvo™ HC-A were the only commercially available human chondrocyte product with gene expression resembling native cells. Moreover, culture on CELLvo™ CD Matrix accelerated proliferation, while minimizing loss of phenotype. Together, these products enable investigators to affordably obtain high quantities of high-quality chondrocytes for research use. In doing so, StemBioSys has eliminated substantial barriers to effective translational research aimed at cartilage repair and regeneration.
References
1. Mao, Y. et al. Extracellular matrix derived from chondrocytes promotes rapid expansion of human primary chondrocytes in vitro with reduced dedifferentiation. Acta Biomat, Accepted.
2. Chen, X-D. et al. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. JBMR, 2007.
3. Pei. M, He, F. Extracellular matrix deposited by synovium derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation. J Cell Physiol, 2012.
4. Marinkovic, M. et al. One size does not fit all: developing a cell-specific niche for in vitro study of cell behavior. Matrix bio, 2016.
StemBioSys® and CELLvo™ are trademarks of StemBioSys, Inc. Methods described in this bulletin may be protected by one or more of the following patents (US 8,084,023; 8,388,947; 9,617,511).