Pancreatic islet transplantation is a surgical procedure used in the treatment of patients with type 1 (or latter-stage type 2) diabetes. It involves transplanting islets of Langerhans (hormone-producing pancreatic cell clusters) from a donor pancreas to the patient’s pancreas, with the goal of regulating blood glucose levels and minimizing hypoglycemia and diabetic complications.
HepatoMatrix: Maturing Hepatocytes For in Vitro Hepatotoxicity Testing
The liver is one of the more complex organs in the human body, and performs many essential functions, including digestion and drug metabolism. In addition to being complex, the liver is also extremely adaptable and can regenerate itself if damaged. Hepatocytes account for about 60% of the cells within the liver, and are responsible for most of its metabolic activity, detoxification, protein secretion and bile production.
CCN1 and the Bone Marrow Mesenchymal Stem Cell Niche
Matrix Biology’s August 2022 edition includes a report entitled “Matrix-bound Cyr61/CCN1 is required to retain the properties of the bone marrow mesenchymal stem cell niche but is depleted with aging”. The report is written by StemBioSys scientific advisor Milos Marinkovic, Ph.D., Assistant Professor in the Department of Comprehensive Dentistry at University of Texas Health at San Antonio. It is based on studies conducted with a team of scientists including StemBioSys CTO Travis Block, Ph.D. and StemBioSys founder and scientific advisor Xiao-Dong Chen, M.D., Ph.D., Professor in the Department of Comprehensive Dentistry at University of Texas Health at San Antonio.
Scientist Spotlight: Anne Sheldrake
Senior Scientist Anne Sheldrake is a key member of the StemBioSys team, with a special focus on developing new products and designing lab protocols. We recently sat down with her to learn more about her background, her day-to-day responsibilities and some important lessons she’s learned over the course of her career.
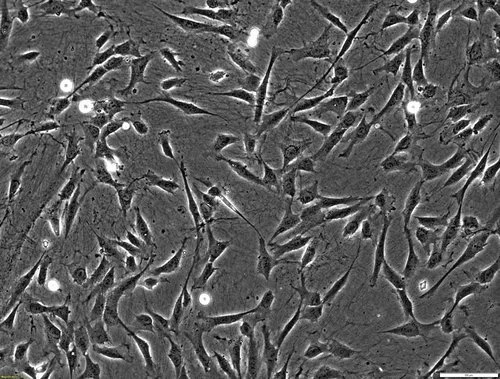
NeuroMatrix™ and Challenges in Neuronal Culture
For scientists studying neurodegenerative disease, investigating new neuroactive drugs, or looking to avoid neurotoxicity in new pharmaceuticals (neurotoxicity is the #1 cause of clinical stage drug attrition), the use of neuronal cell culture shows great promise for modeling aspects of the most complex organ in the body, so that we may gain new insights to advance basic and translational research.
Organoids: Expectations and Realities
The 9th Annual San Antonio Conference on Stem Cell Research & Regenerative Medicine (or RegenMed SA, as it’s commonly known) will convene at the BioBridge Global Campus on February 9-10, 2023. RegenMed SA promotes collaboration among people and organizations interested in stem cell research, tissue engineering and regenerative medicine.
Click Chemistry: What it is and How it Affects Cell-Based Research
Congratulations to Carolyn R. Bertozzi (Stanford University), Morten Meldal (University of Copenhagen) and K. Barry Sharpless (Scripps Institute) - winners of the 2022 Nobel Prize in chemistry. These renowned scientists were awarded the honor for their development of click chemistry and bioorthogonal chemistry.
Phasing Out Animal Testing
The FDA Modernization Act, which was unanimously passed by the Senate in September 2022, seeks to diversify the methods by which drug companies can test their products for safety and efficacy before they are marketed to the public. The passing of this bill represents the culmination of years of research and collaboration between scientists, drug company representatives and various members of the government.
Cartilage Tissue Engineering: a New Frontier in Knee Rehabilitation
Several years ago, The U.S. Food and Drug Administration (FDA) approved a product called MACI® for the repair of knee cartilage defects in adult patients. MACI, an acronym for matrix-induced autologous chondrocyte implantation, is an advanced cell therapy developed and marketed by American biopharmaceutical company Vericel Corporation; it utilizes the groundbreaking technique of cartilage tissue engineering to address knee damage resulting from injury, overuse and surrounding muscle weakness.